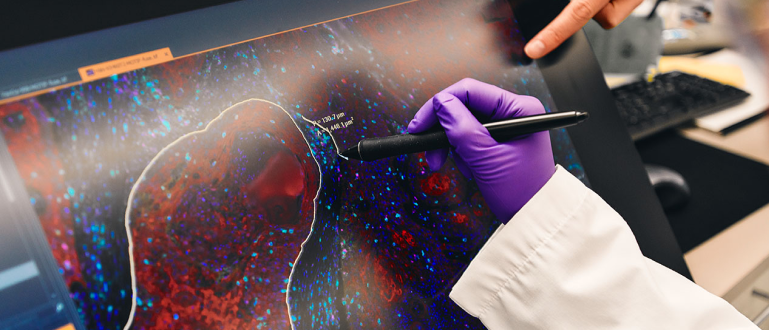
藥物開發
加速科學發展
在 BMS,每天在早期和後期發展的不同治療領域中,您都會受到有趣和有意義的工作啟發。我們透過臨床試驗、數據科學和策略營運,引領創新,為患者提供一流的藥物和療法。
在整個藥物開發階段都有機會能為您帶來挑戰。工作內容並不容易,但所做的事獨特又有趣。在這裡,您將改寫規則、解決最艱難的挑戰,並創造奇蹟。
Guiding the Vision for Drug Development
Living the mission of transforming patients’ lives through science, Drug Development leadership continue to inspire and be inspired by the incredible work happening now:

Cristian Massacesi, MD

Jennifer Dudinak

Mariette Boerstoel

Michelle Hudson

Mokash Sharma

Nataraj Kalyanaraman

Zhengying Zhu
藥物開發職涯
BMS 的藥物開發部門提供多元化的職位,需要各種技能和專業知識。無論您對開發、數據科學、患者安全性或策略營運感興趣,都有機會為新藥的開發做出貢獻。
血液學、腫瘤學、細胞療法發展
為治療癌症和血液疾病(包括細胞療法)等藥物開發工作做出貢獻。
免疫學、心血管、神經科學發展
支持免疫學、心血管和神經科學藥物的開發,好讓我們能為患者提供最佳療法。
全球生物辨識和數據科學
利用資料和機器學習推動定量決策、加速藥物開發並改善患者結果。
全球發展營運
利用營運科學成功地提供我們的藥物產品組合。
全球監管科學
透過創新的全球監管策略和卓越的營運,合作為患者提供藥物,同時塑造監管科學和政策的未來。
Portfolio & Strategic Operations
管理我們的產品組合,優化和最大化公司的價值。
全球患者安全性
在整個生命週期中監測產品安全性,將對患者的風險和傷害降至最低。
Our Areas of Focus
Development Hematology, Oncology, Cell Therapy (HOCT)

- Development Hematology, Oncology, Cell Therapy (HOCT)
- Development Immunology, Cardiovascular, Neuroscience (ICN)
- Global Biometrics & Data Sciences
- 全球發展營運
- 全球監管科學
- 產品組合與策略營運
- 全球患者安全性
Development Hematology, Oncology, Cell Therapy (HOCT)

Development Immunology, Cardiovascular, Neuroscience (ICN)
Global Biometrics & Data Sciences
全球發展營運
全球監管科學
產品組合與策略營運

全球患者安全性


我們在印度海德拉巴的技術與創新中心
BMS 海德拉巴是一處整合性全球研究中心,旨在透過建立永續且創新的解決方案,從而幫助患者戰勝嚴重疾病。我們在海德拉巴的發展部門,將協助建立重要能力來支援藥物生命週期的多個重要階段,包括監管、生物統計學、營運和臨床科學。這項工作將進一步加速我們的藥物開發,並促進 BMS 發現、開發和提供創新藥物以幫助患者的使命。
職涯故事
聽聽我們世界各地藥物開發團隊成員的故事,瞭解他們每天為患者所做的工作。







