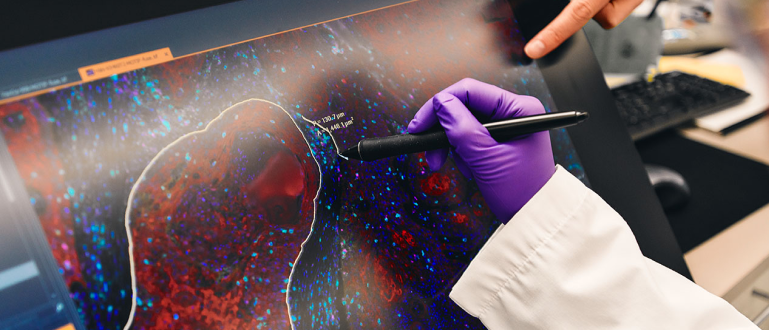
药物开发
加速推进科学发展
在 BMS,你每天都会因我们治疗领域早期和后期开发中既有趣又有意义的工作而受到鼓舞。通过临床研究、数据科学和我们的战略运营,我们引领创新,为患者提供创新药物和疗法。
在药物开发的各个阶段,你会获得丰富的挑战机会。它绝不简单,但绝对有趣。你将在这里改写规则、应对艰巨挑战并创造奇迹。
Guiding the Vision for Drug Development
Living the mission of transforming patients’ lives through science, Drug Development leadership continue to inspire and be inspired by the incredible work happening now:

Cristian Massacesi, MD

Jennifer Dudinak

Mariette Boerstoel

Michelle Hudson

Mokash Sharma

Nataraj Kalyanaraman

Zhengying Zhu
药品开发职业发展机会
BMS 药物开发部门为具备相关技能和专业知识的人才提供丰富的职业发展机会。无论你是对开发、数据科学、患者安全,还是战略运营感兴趣,都有机会为新药开发做出贡献。
血液学、肿瘤学、细胞治疗领域开发
为癌症和血液疾病治疗领域(包括细胞治疗)的药物开发工作做贡献。
免疫学、心血管、神经科学领域开发
为免疫学、心血管和神经科学领域的药物开发提供支持,向患者提供最佳治疗方法。
全球生物统计与数据科学
利用数据和机器学习技术推动定量决策、加速药物开发,改善患者治疗结果。
全球开发运营
利用运营科学成功将我们的系列药品推向市场。
全球注册科学
通过全球创新注册策略和卓越运营建立合作关系,为患者提供药品,同时塑造未来科学监管与政策。
Portfolio & Strategic Operations
妥善管理我们的产品组合,使公司的价值得到优化和最大化。
全球患者安全
在整个周期中监控产品安全,尽量减少对患者的风险和伤害。
Our Areas of Focus
Development Hematology, Oncology, Cell Therapy (HOCT)

- Development Hematology, Oncology, Cell Therapy (HOCT)
- Development Immunology, Cardiovascular, Neuroscience (ICN)
- Global Biometrics & Data Sciences
- 全球开发运营
- 全球注册科学
- 产品组合与战略运营
- 全球患者安全
Development Hematology, Oncology, Cell Therapy (HOCT)

Development Immunology, Cardiovascular, Neuroscience (ICN)
Global Biometrics & Data Sciences
全球开发运营
全球注册科学
产品组合与战略运营

全球患者安全


我们位于印度海得拉巴 (Hyderabad) 的技术与创新中心
BMS 海得拉巴的综合性全球中心,致力于通过建立可持续创新解决方案,帮助患者战胜严重疾病。我们在海得拉巴的研发组织将帮助建立重要能力部门,为药物开发周期中的注册、生物统计学、运营和临床科学等重要阶段提供支持。这项工作将进一步加速我们的药物开发并推动 BMS 践行使命,发现、开发和提供惠及患者的创新药物。
职业故事
看看我们世界各地的药物开发团队成员每天如何为患者工作。







